9 Standard Plate Count
In certain circumstances, we need to know the concentration of bacteria in a sample. For instance, the count of bacteria might indicate the extent of infection occurring, such as in a bladder infection. Quality control has to depend on the numbers of bacteria present in items like drinking water, food, medication, cosmetics and even swimming areas. Different methods of determining the number of bacteria are used, but each method has its own advantages and disadvantages. We are going to use the standard plate count method. It is a direct method of counting viable, or live, cells, which is its advantage. The standard plate count is where a sample is diluted in a series of dilution blanks, and then aliquots (measured amounts) of the dilutions are plated using either the pour plate method or spread plate method. (See Figure 9.1). The expected result is a single bacterial cell gives rise to a single bacterial colony. However, this is not true for bacteria that do not immediately separate after dividing, so multiple cells could form a well-isolated colony. A more accurate unit for counting is a colony forming unit (cfu). The number of bacterial cfu in the original sample is determined by multiplying the plate count by the dilution factor of the plate that is being counted. To be statistically accurate and effective, only numbers between 30 and 300 cfus are considered. Fewer than 30 cfus makes the statistics invalid due to a random noise associated with the probability a cfu will end up on a plate. At higher numbers, this noise is a small percentage of the count. Greater than 300 cfus could be inaccurate due to overcrowding inhibiting the growth of possible colonies and imprecision of counting.
Disadvantages of standard plate count are 1) the time it takes to obtain results due to the incubation period for the colony forming units to grow and 2) the limitation of what bacteria can be analyzed. The method uses specific media and conditions. Pour plates do not limit oxygen diffusion significantly, but the method may be beneficial in cases of bacteria species that grow poorly in air (microaerophiles). For aerobes, the minor oxygen limitation from metabolic activity slows growth and keeps colonies small. Selective medium is used when determining bacterial number for a urinary tract infection.
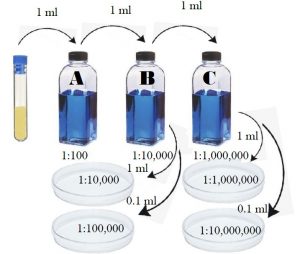
Figure 9.1 Quantitative plate procedure of standard plate count.
MATERIALS
Per pair of students should have:
Blue rack
Wax pencil
3 sterile 99 ml water blanks
7 sterile 1 ml serological pipettes
2- 1 ml blue pi-pumps
1 broth culture of E. coli
4 empty sterile petri plates
4 pour tubes (remain in hot water bath until ready to use)
(OPTIONAL: Bunsen Burner & Striker)
PROCEDURE OF SERIAL DILUTION AND PLATING
- Label three sterile 99 ml water blanks A, B, and C.
- Label the bottom of the four sterile petri plates 10-4, 10-5, 10-6, and 10-7. On each plate also include your names, bacteria name, and date.
- Gently flick the culture tube to disperse the bacteria.
- Transfer 1 ml of E.coli from the culture tube into blank A using a sterile 1 ml pipette. Place the used pipette into the small biohazard bag on the lab bench.
- Shake blank A 25 times in an arc of 1 foot in order to distribute the bacteria evenly. Do not let the bottle go upside down. (Suggestion: place a paper towel over the lid of the water blank while shaking. If any leakage onto the paper towel, place that paper towel into the biohazard bag near the prep room door.)
- Using a different sterile 1 ml pipette, transfer 1 ml from blank A into blank B.
- Shake blank B 25 times in an arc of 1 foot in the same manner.
- Using a different sterile 1 ml pipette, transfer 1 ml from blank B into blank C.
- Shake blank C 25 times in the same manner.
- Using a different sterile 1 ml pipette, transfer 1 ml from blank B into plate 10-4 plate.
- Using a different sterile 1 ml pipette, transfer 0.1 ml from blank B into plate 10-5 plate.
- Using a different sterile 1 ml pipette, transfer 1 ml from blank C into plate 10-6 plate.
- Using a different sterile 1 ml pipette, transfer 0.1 ml from blank C into plate 10-7 plate.
Dilution scheme
| 99 mL + | 1 mL from | To plate 10-4 | To plate 10-5 | To plate 10-6 | To plate 10-7 |
| Blank A | Original | ||||
| Blank B | Blank A | 1 mL | 0.1 mL | ||
| Blank C | Blank B | 1 mL | 0.1 mL |
14. Taking the blue rack with you, go to the water bath and remove 4 pour tubes, and bring back to your lab bench.
15. Pour one tube of liquefied Trypticase Soy Agar into each plate, cover with the plate lid, and swirl gently. Push the plates slowly and gently towards the center of the lab bench to solidify.
16. Under the instruction of your lab professor, you will move the plates at a later time during the lab session to your lab section’s storage location to be stored at room temperature.
17. Place empty pour tubes, E.coli culture tube, and used blank bottles in the Discard Area. Return the blue pi-pumps back to its location. Please make sure the rubber pi-chuck is removed from used pipettes if it became removed from the blue pi-pump.
During next week’s lab period, you will examine the results of these plates to determine how many bacterial cells are in one milliliter of the original culture of E. coli.
REFERENCES
Lumen Learning. (n.d.). Counting Bacteria. Retrieved from Boundless Microbiology: https://courses.lumenlearning.com/boundless-microbiology/chapter/counting-bacteria/
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
OpenStax. (2016, November 1). Microbiology. Retrieved from https://legacy.cnx.org/content/col12087/1.4
