3 Streak Plate Method
You have learned that microbes are found everywhere and coexist with each other in mixed populations as well as with larger organisms such as you. To observe and study bacteria, you have to isolate individual bacterial species into pure cultures. A pure culture contains only a single kind of species whereas a mixed culture contains more than one species in one medium. (Figure 3.1). A contaminated culture contains the desired species but also unwanted species. In a contaminated culture it is hard to tell which bacteria are performing which function. These contaminants can outgrow the bacteria you are interested in studying.
Several methods of achieving pure culturing are used in the microbiology lab. Two commonly used ones in our lab are the streak plate and the pour plate methods. These involve diluting bacterial cells in a sample down to an end point of a single genotype dividing and giving rise to a single pure colony. A colony is a collection of identical cells of the same bacterial strain, growing on or within a solid medium. A streak plate is the most economical in time and materials: one medium plate and a quick inoculation. However, streaking is a procedure that takes practice to reliably large numbers of well-separated, individual colonies. For the streak plate method, one loopful of bacterial culture is diluted by streaking it onto the surface of an agar plate, making sure the cells are spread out. The pour plate method consumes more time and more materials. A standard pour plate method is a serial dilution of one loopful of a bacterial culture being diluted in a series of tubes of liquefied agar. After the dilution step is finished, each liquefied agar tube is poured into a sterile empty petri plate. Bacterial colonies will grow on and within the agar after it solidifies. These other methods have the advantage of quantifying the original culture density. For this exercise, you will do the streak plate. There are several methods of performing the streak plate. Each student will do 2 T streak method plates.
| MATERIALS |
 |
| Each student should have: | |
| Blue rack | |
| 4 sterile TSA plates (Trypticase Soy Agar) | |
| Inoculating loop | |
| Wax pencil | |
| Bunsen Burner | |
| Striker
1 culture of Staphylococcus epidermidis |
|
| 1 culture of Escherichia coli
|
|
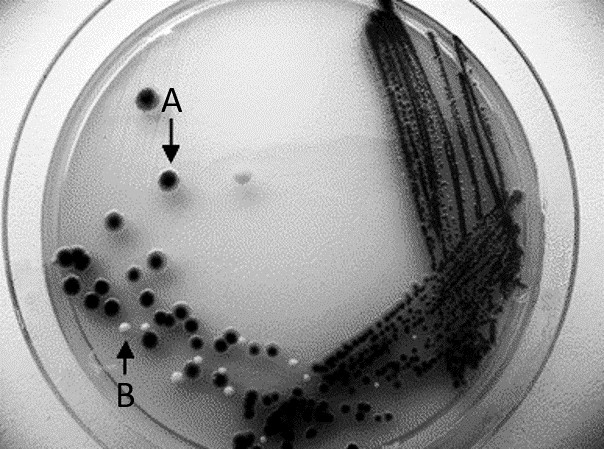
| Figure 3.1: Streak plate method can create isolated colonies needed for a pure culture. Dark colony (A) can be streaked on another plate to produce pure culture without the light-colored contaminant (B). | |
PROCEDURE
T Streak: (Figure 3.2)
- Label the bottom of the TSA plate along the edge of the plate with your wax pencil: your name, bacteria name, and date.
- Create a large “T” along the whole bottom of the plate. This divides the plate into three sections: 1, 2, and 3.
- Using aseptic technique, transfer one loopful of bacteria onto section 1 (above the “T”) by streaking onto the surface of the agar plate.
- Flame the loop, and then stab into a sterile area of the plate to cool.
- Streak from section 1 into section 2. Do not backtrack.
- Flame the loop, and then stab into a sterile area of the plate to cool.
- Streak from section 2 into section 3. Do not backtrack.
- Flame the loop to sterilize when finished.
- Store plates upside down in your lab section’s rack at room temperature.
Section 1 Section 2 Section 3
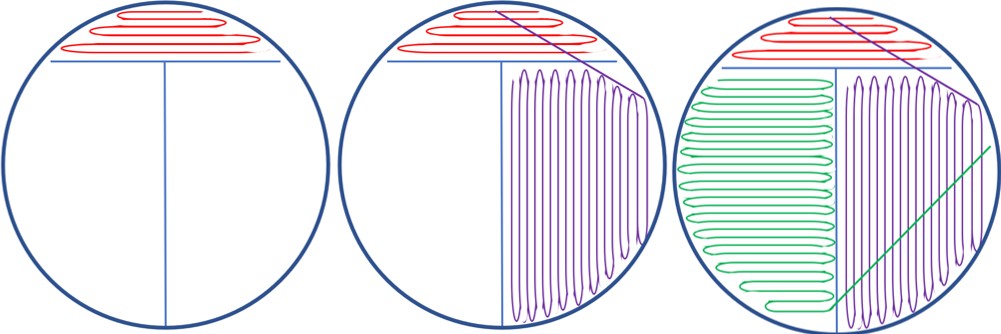
Figure 3.2: T streak method is literally drawing the letter “T” on the bottom of the plate to make it clear where to drag the loop. Use a single loopful of bacteria in section 1 and dilute down into the section following. Make sure to flame the inoculating loop between each streak. It helps to rotate the petri plate counterclockwise while you are doing the streak plate method. You can improve your ability to get sufficient bacteria into a new section by pulling bacteria from the first stroke in the previous section. Pull across your previous strokes by using the light reflecting from the agar surface to visualize your previous strokes.
REFERENCES
Biology Online . (2001). Retrieved from https://www.biology-online.org/dictionary/Colony
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
Chess, B. (2015). Laboratory Applications in Microbiology: A Case Study Approach. New York: McGraw Hill.
