11 Antimicrobial Sensitivity Testing: Kirby Bauer Disk of Diffusion Method
A general term used is antimicrobial, which is a compound that inhibits or kills microorganisms. There are different types of antimicrobials. Examples are antibiotics, disinfectants, and antiseptics. Antibiotics are produced by microorganisms that inhibit or kill other microorganisms by targeting specific cellular processes. Antibiotics shut down specific cellular processes, so their mode of action affects specific kinds of cells. Disinfectants and antiseptics don’t have that specificity. They destroy structures in a wide range of cells, including your own. Because of this, Disinfectants are used to destroy or inhibit microbial cells on inanimate objects. A subgroup of disinfectants, antiseptics, kill or prevent growth for microbes on human tissue. They are also not specific in what cells they affect, but they only affect your dead outer layer of cells.
A procedure named the Kirby Bauer Disk of Diffusion Method finds the suitability of specific antibiotics for specific infections. This method consists of growing a uniform layer of bacteria on a solid medium (known as a lawn). A small filter disk, saturated with a specific concentration of antibiotic, is place on that plate. During incubation, the antibiotic will diffuse from the disk into the agar, forming a concentration gradient. The level of sensitivity to the particular agent can be determined by measuring a clearing around the disk where the bacteria did not grow. This clearing is known as the Zone of Inhibition (ZoI). Its size varies for the agent as a result of the resistance of the bacteria. The size of the ZoI can be used to decide the effectiveness of the antibiotic. A larger ZoI indicates that the bacteria are more sensitive to the antibiotic. But the simple presence of a ZoI does not mean that the antibiotic will work for treating a patient. Kirby Bauer method is a standardized test, and has minimum ZoI diameters for each antibiotic that varies by bacterial type involved. These diameters are related to the probable clinical outcomes for patients. The different criteria for different bacterial types are primarily based on the efficiency of delivering the drug to the typical infection site for a bacterial type. For instance, a pill dissolves in the gut enters the blood and perfuses tissues from every accessible side. Skin and lung sites have no blood flow on the side away from the tissues. So, skin and lung infections will tend to have higher ZoI diameter criteria for sensitivity than gut infections, so that even with decreased perfusion the infection will still be successfully treated. Test results are reported as sensitive, resistant, or intermediate. (Table 11.1). If the zone of inhibition is greater than the standard size zone of inhibition, then the bacteria is sensitive to the antibiotic. If the zone of inhibition is smaller than a specific ZoI diameter, then the bacteria is resistant to the antibiotic, meaning that antibiotic is not effective on that bacteria. Physicians can isolate and identify the bacteria causing the infection in the patient and use the Kirby Bauer method to see which antibiotic would be most effective.
Using 95% ethanol in a glass petri dish
Part of the effectiveness of flame sterilization is the mix of free-radicals that aggressively react with the cells. Ethanol increases the range of free-radicals, allowing for lower-heat sterilization.
MATERIALS
Each pair of students should have:
Wax pencil
2 media plates: depending on availability:
MH plate (Mueller Hinton) and 1 TSA plate (Trypticase Soy Agar)
OR
2 TSA plates
2 Sterile swabs
Sterile forceps
Bunsen Burner
Striker
Disinfectants: hydrogen peroxide, 5% bleach, 5% formalin, Lysol
Antibiotics: Choose 3
a) Penicillin d) Tetracycline
b) Cephalothin e) Erythromycin
c) Chloramphenicol f) Ampicillin
1 broth culture of bacteria of instructor’s choosing for your pair for equal distribution:
Staphylococcus epidermidis
Klebsiella aerogenes
Escherichia coli
At your station: 95% ethanol in a glass petri dish
Sterile filter disks
PROCEDURE OF KIRBY BAUER DISK OF DIFFUSION METHOD
- Label the bottom of each plate with student names and date. On the MH plate with a wax pencil, draw three lines to divide into thirds. On the TSA plate, draw two lines to make quadrants. Label as follows:
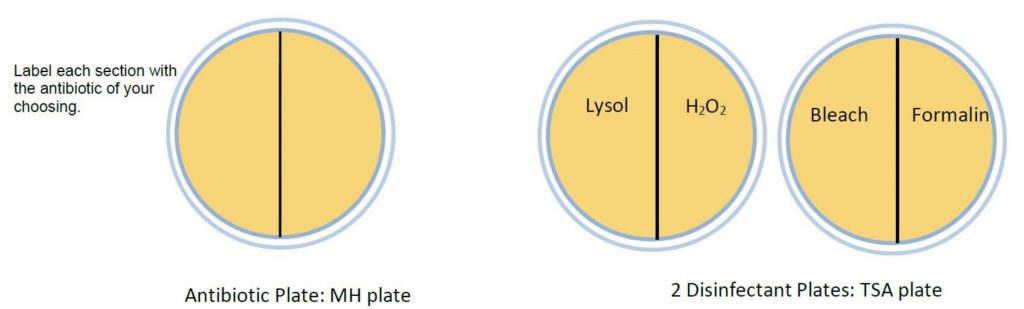
- Using aseptic technique, make a lawn of bacteria on each plate by inserting a sterile cotton swab into the broth bacterial culture, absorbing excess fluid. Gently and evenly spread the moistened swab over the surface, making sure you cover the entire surface. In order to create thorough lawn, rotate the plate counterclockwise twice, continuing to spread the swab on the surface between each turn.
- Use a separate sterile swab for each plate.
- Allow the plates to dry for a few minutes before applying disks.
- At each station, there are two glass petri dishes: a) 95% ethanol and b) sterile filter disks.
- Disinfectants: Dispense disks as follows:
a. Dip forceps into 95% ethanol and then place into outer flame of Bunsen Burner until red. Allow to cool for a few seconds by waving in the air.
b. Carefully remove a sterile filter disk from the glass plate (do not put the lid down on the bench) with the forceps.
c. Saturate the disk with one drop of 5% bleach.
d. Place the disk in the center of the quadrant on the TSA plate labeled “5% bleach”.
e. Dip forceps into 95% ethanol and then place into outer flame of Bunsen Burner until red. Allow to cool for a few seconds by waving in the air.
f. Repeat steps a through e for the next three disinfectants: hydrogen peroxide, 5% formalin, and Lysol. - Antibiotics: Dispense disks as follows:
a. Dip forceps into 95% ethanol and then place into outer flame of Bunsen Burner for a few seconds. Allow to cool for a few seconds by waving in the air.
b. Carefully push half of the antibiotic disk out of the cartridge with the forceps, and then once half is available, pull the disk out with the forceps.
c. Place the disk in the center of a section labeled with the appropriate antibiotic.
d. Dip forceps into 95% ethanol and then place into outer flame of Bunsen Burner until red. Allow to cool for a few seconds by waving in the air.
e. Repeat steps a through d for the next two antibiotics of your choosing.
Incubate your plates at room temperature in your lab section’s racks.
DETERMINING RESULTS (WEEK 2)
1. Measure the diameter of the zone of inhibition of each antimicrobial, specifically the antibiotics, using a small metric ruler in millimeters. Simply use the naked eye for the determination of the zone of inhibition.
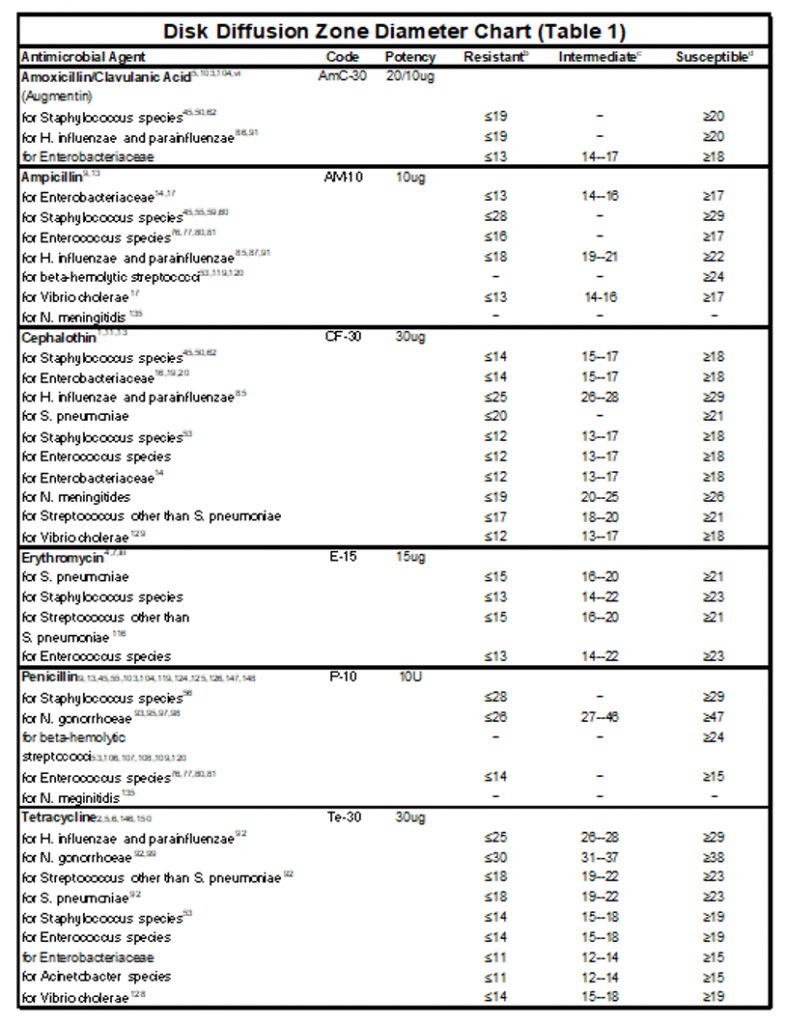
2. You can determine if your bacteria is resistant (R ), sensitive (S), or intermediate to the antibiotics by looking at Table 11.1. The zone of inhibition varies between different bacteria and different antibiotics.
Table 11.1: Zones of inhibition in the Kirby Bauer Disk of Diffusion Method of Antibiotic Sensitivity Testing.
REFERENCES
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
Cowan, M. K. (2015). Microbiology: A Systems Approach (4th edition). New York: McGraw Hill.
Hardy Diagnostics. (2015). Instructions for Use. Retrieved from HardyDisk TM Antimicrobial Sensitivity Test: https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/HardyDiskASTProceduresandChart.pdf
Lumen Learning. (n.d.). Measuring Drug Susceptibility. Retrieved from Boundless Microbiology: https://courses.lumenlearning.com/boundless-microbiology/chapter/measuring-drug-susceptibility/
