13 Growth Requirements of Aerobes versus Anaerobes
Not all bacteria can tolerate oxygen, and many actually need to be in an environment without it in order to survive the oxidation. It is very useful to classify into groups based on their response to oxygen or of the lack of oxygen.
Bacteria that require oxygen for growth are called aerobes. They carry out respiratory metabolism, or aerobic respiration, where oxygen is the terminal electron acceptor. There are different types of aerobes: obligate aerobes and microaerophiles. Obligate aerobes absolutely require oxygen for growth for aerobic respiration to be carried out. By this process, they yield a great deal of energy compared to anaerobic bacteria. Microaerophiles are bacteria that live at lower concentrations of oxygen than atmospheric concentration, such as in the soil, water, or the human vagina. They prefer approximately 10% oxygen concentration rather than 20%. They have a limited capacity to neutralize reactive oxygen species (toxic byproducts of oxygen and oxidation). Examples of the kinds of enzymes they might lack are peroxidase, catalase, and superoxidase dismutase.
Bacteria that are sensitive to oxygen are called anaerobes. Some are more sensitive than others. The types of anaerobes are: facultative anaerobes, aerotolerant anaerobes, and obligate anaerobes. Facultative anaerobes can carry out fermentation or anaerobic respiration if oxygen is not present. But it can also carry out aerobic respiration if oxygen is present. The reason for this is that these bacteria possess the detoxifying enzymes mentioned previously. Aerotolerant anaerobes are a subgroup of facultative anaerobe. They can carry out only fermentation if oxygen is not present. They do not carry out aerobic respiration if oxygen is present, but they are not harmed by its presence in the environment. Instead of possessing the detoxifying enzymes, aerotolerant anaerobes contain metallic ions that have the same function. Obligate anaerobes are bacteria that cannot tolerate oxygen due to having the sensitivity to the byproducts of oxygen, such peroxides and superoxides. They do not possess the detoxifying enzymes. They carry out fermentation or anaerobic respiration. Because of their sensitivity to the byproducts of oxygen, anaerobes have to be grown in an oxygen-free environment. Different methods have been developed, but we will be using either one of two ways to do so: fluid thioglycollate medium or a GasPak anaerobic jar.
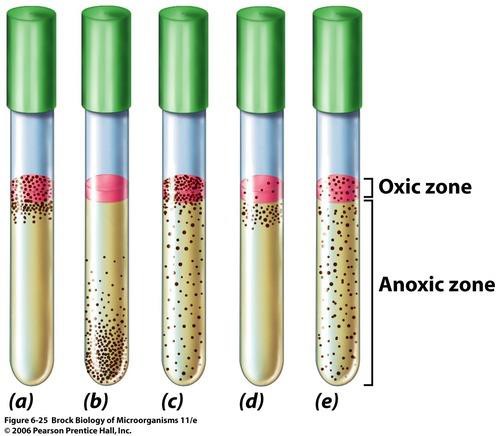
Figure 13.1 (a) Obligate aerobe (b) Obligate anaerobe (c) Facultative anaerobe (d) Microaerophile (e ) Aerotolerant anaerobe
Fluid thioglycollate medium is a liquid medium that supports growth of aerobes and anaerobes. (Figure 13.1). It contains sodium thioglycolate, which chemically combines with dissolved oxygen to deplete the oxygen in the medium. It also prevents the accumulation of peroxides. It contains an oxidation-reduction (redox) indicator named resazurin. It turns pink when oxidized, and is colorless when reduced. A thin band is more desirable because it is fresher. There is a small amount of agar in the medium that assists in the maintenance of low redox potential: it keeps the anaerobes at the bottom of the tube.
An anaerobic jar becomes an anaerobic environment by using a GasPak sachet. The GasPak contains inorganic carbon, activated carbon, and ascorbic acid. Once the sachet becomes activated by simply opening it, it creates an oxygen-free and carbon dioxide-rich environment inside the sealed anaerobic jar. (Figure 13.2).

|
Figure 13.2 The GasPak sachet contains chemicals to release carbon dioxide and hydrogen gas. The hydrogen gas binds with free oxygen in the jar, producing water. This can be seen by the condensation in the inside of the jar |
This lab will focus on examples of obligate aerobe, facultative anaerobe, and obligate anaerobe.
2 options of the experiment based on availability of materials: Fluid thioglycollate Medium Or GasPak Anaerobic Jar
For Fluid Thioglycollate Medium Experiment:
MATERIALS
Per group of 3 students should have:
3 test tubes of fluid thioglycollate medium
3 test tubes (one per above test tube)
Wax pencil or their own pen or pencil
3 Inoculating loops (one per student)
Bunsen Burner
Striker
Example set of species:
Clostridium sporogenes
Escherichia coli
Micrococcus luteus
PROCEDURE OF FLUID THIOGLYCOLLATE MEDIUM
- Each student is responsible for one bacterial culture being transferred.
- Label each fluid thioglycollate medium tube with your name, date, and the bacterial culture in which you are working with.
- Using aseptic technique, transfer 3 loopfuls of the bacterial culture to a properly labeled sterile fluid thioglycollate medium tube.
- Place the 3 freshly inoculated tubes into your lab section’s red racks at room temperature.
For GasPak Anaerobic Jar Experiment:
MATERIALS
Per pair of students should have:
2 TSA plates (Trypticase Soy Agar)
2 Inoculating loops (one per student)
2 wax pencils
Bunsen Burner
Striker
Example set of species:
Clostridium sporogenes
Escherichia coli
Micrococcus luteus
Per class:
One Gas Pak sachet
One Anaerobic Jar
One Regular Plate Rack
PROCEDURE OF GASPAK ANAEROBIC JAR
Each student is responsible for one plate.
- Label the bottom of each TSA plate with your names and date. Label as follows:
- Using aseptic technique, use a loop to inoculate each plate with a single streak of each bacterial directly under their label.
- Place one plate in the anaerobic jar upside down and the other in the regular rack upside down to be incubated at the normal oxygen concentration.
For the instructor:
- Once all plates have been collected for the class, rip open the GasPak, and remove it from the silver envelope and place it inside the anaerobic jar.
- Screw the anaerobic jar shut.
- The jar will be oxygen free within 2½ hours and greater than 15% carbon dioxide within 24 hours.
- Place both the anaerobic jar and the regular plate rack in your lab section’s area on the lab bench at room temperature in order to observe results next week.
REFERENCES
BD (TM) Fluid Thioglycollate Medium. (2003, August). Retrieved from Becton Dickinson Systems: http://www.bd.com/europe/regulatory/Assets/IFU/HB/CE/BA/BA-257144.pdf
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
Chess, B. (2015). Laboratory Applications in Microbiology: A Case Study Approach. New York: McGraw Hill. Cowan, M. K. (2015). Microbiology: A Systems Approach (4th edition). New York: McGraw Hill.
GasPak EZ Anaerobe Sachets with Indicators. (2018). Retrieved from Carolina Biological: https://www.carolina.com/catalog/detail.jsp?prodId=801420&s_cid=ppc_gl_products&utm_source=go ogle&utm_medium=cpc&scid=scplp801420&sc_intid=801420&gclid=EAIaIQobChMItN6tz4_E2AIVxiOBC h1mhA6NEAQYASABEgJXovD_BwE
Madigan, M. and John Martinko. (2006) Brocks Biology of Microorganisms (11th edition). San Francisco: Benjamin Cummings.
