12 Lethal Effects of Ultraviolet Light
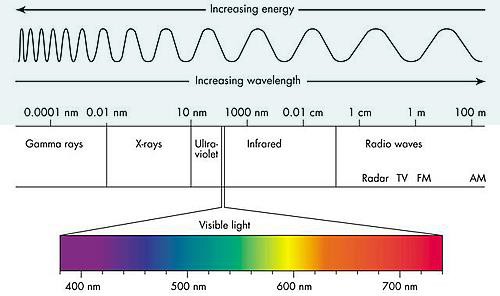
Electromagnetic spectrum is the range of wavelengths of electromagnetic radiation (or energy), extending from the longest wavelength of radio waves to the shortest of gamma rays. The two forms of electromagnetic radiation that are mutagenic are ionizing radiation and nonionizing radiation. Ionizing radiation carries enough energy that electrons are ejected and ions are formed which breaks DNA strands and denature proteins. Examples of ionizing radiation are gamma rays and x rays. Nonionizing radiation just excites electrons to a higher energy state. This includes visible light. (Figure 12.1). Shorter wavelengths can be absorbed by molecular bonds, the way sunscreen or a black-light poster absorb UV. UV is absorbed by the ring-structure bonds of pyrimidines. The electrons are excited to a higher energy state and interact with excited electrons from the neighboring pyrimidine. Abnormal bonds are formed in DNA. Pyrimidine dimers occur when two adjacent pyrimidines (particularly thymine) form covalent bonds between their ring-structures. (Figure 12.2). An example of nonionizing radiation is ultraviolet light. The wavelength of ultraviolet spans between 4 and 400 nm, but the most germicidal wavelength is 260 nm due to this being the wavelength that DNA maximally absorbs ultraviolet light, causing the pyrimidine dimer formation. (Figure 12.3). Genes with pyrimidine dimers are damaged and cannot be transcribed of replicated. This may be lethal.
 |
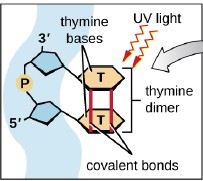 |
| Figure 12.1 Electromagnetic spectrum illustrates how the shorter the wavelength, the more energy, therefore the more damaging to cells. (http://www.astro.wisc.edu/~kerry/pics/em.jpg) | Figure 12.2 Ultraviolet light cause the formation of abnormal bonding of adjacent thymine or cytosine: pyrimidine dimer.
(OpenStax, Microbiology p473) |
Several elements affect the killing properties of UV light, including the time of exposure to the ultraviolet light and the presence of materials that will block the radiation from reaching the cells. An example of this is the plastic lid of the petri plate. Endospores are more resistant than vegetative cells to UV light. The DNA of endospores is protected by small, acid-soluble proteins that either absorb UV or promote a DNA conformation that avoids pyrimidine dimer formation.
Repair is very expensive, particularly at lower levels of light (Figure 12.4). When the cost of repair exceeds the available resources of the cell, then damage is not repaired and new enzymes for energy and repair cannot be produced if the relevant genes are damaged with pyrimidine dimers. “Death by UV equals debt by UV.”
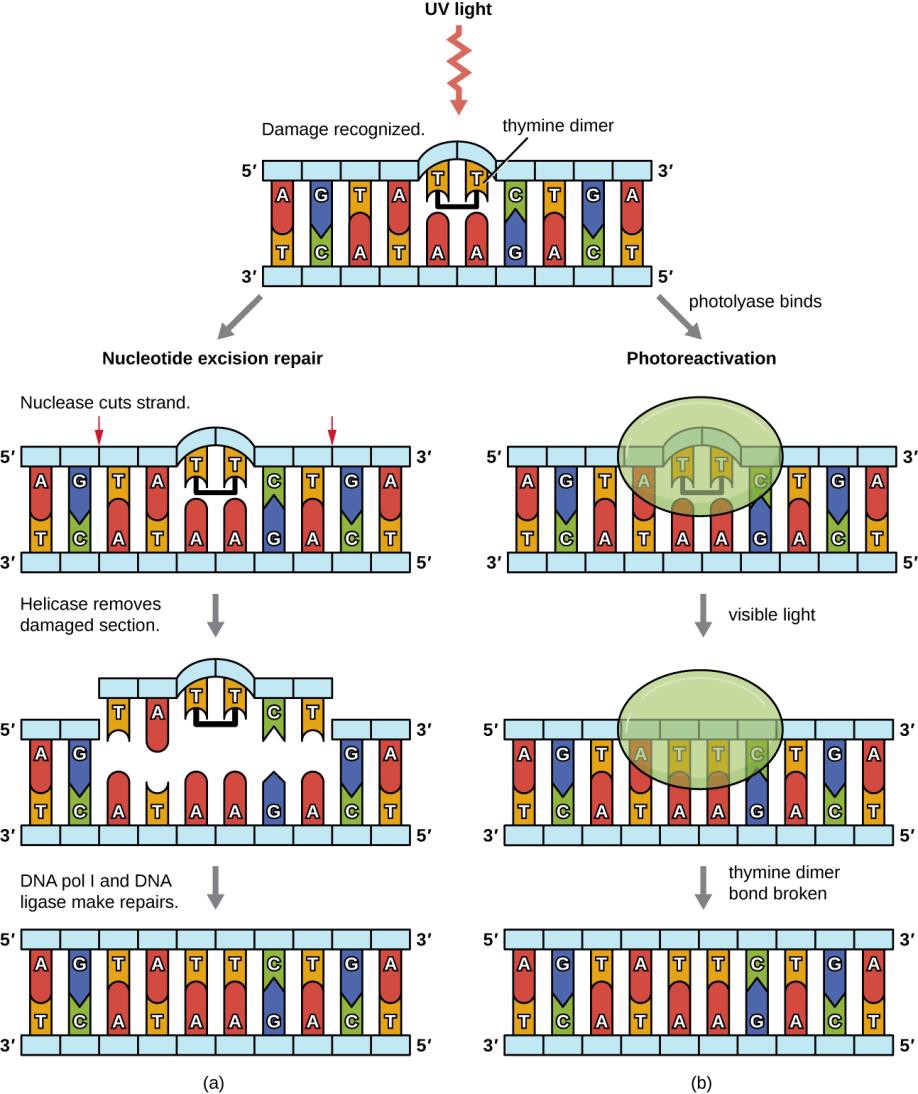
Figure 12.4 (OpenStaxMicrobiology, Figure 11.23) Bacteria have two mechanisms for repairing thymine dimers. (a) In nucleotide excision repair, an enzyme complex recognizes the distortion in the DNA complex around the thymine dimer and cuts and removes the damaged DNA strand. The correct nucleotides are replaced by DNA pol I and the nucleotide strand is sealed by DNA ligase. (b) In photoreactivation, the enzyme photolyase binds to the thymine dimer and, in the presence of visible light, breaks apart the dimer, restoring the base pairing of the thymines with complementary adenines on the opposite DNA strand.
This experiment is going to compare the germicidal effects of ultraviolet light on endospore former bacteria (Bacillus subtilis) to two non-endospore former bacteria (Escherichia coli and Staphylococcus epidermidis).
MATERIALS
Per student:
Blue rack
Sterile cotton swab
Wax pencil
Index card
1 TSA plate (Trypticase Soy Agar)
Safety glasses
Pair of nitrile gloves
1 broth bacterial culture of Escherichia coli or Staphylococcus epidermidis or Bacillus subtilis (resuspended from a slant)**
(Optional: Bunsen burner & striker)
PROCEDURE OF ULTRAVIOLET LIGHT LAB
- The instructor will distribute one cultural tube per student, and assign an exposure time to each student.
- Label the bottom of the TSA plate with your name, date, the bacterial culture, and exposure time in which you are working with.
- On the bottom, draw a line down the middle of the plate, dividing it in half. Label one side “UV” and the other “No UV”.
- Using aseptic technique, make a lawn of bacteria on each plate by inserting a sterile cotton swab into the broth bacterial culture, absorbing excess fluid. Gently and evenly spread the moistened swab over the surface, making sure you cover the entire surface. In order to create thorough lawn, rotate the plate counterclockwise twice, continuing to spread the swab on the surface between each turn.
a. **Students who have a Bacillus subtilis slant will need to resuspend the colonies into a liquid: Using a sterile swab, swipe the culture off of the slant and put that swab into a sterile water bottle. Shake the swab inside the water until the bacteria is removed off of the swab. (Or add sterile water to the slant. Screw closed and vortex.)
b. Otherwise follow your instructor’s directions to resuspend. - Wearing safety glasses and nitrile gloves, bring your TSA plate and index card to the ultraviolet light station.
- Remove the lid from the petri plate, and cover the half of the plate labeled “No UV” with the index card.
- Expose your plate to the UV light for your assigned exposure time. You can use the second hand on the clock or the timer on your cell phone to time the exposure.
- Place your finished plate with its lid on upside down in a rack in your lab section’s area.
For the instructor:
Once the entire class has completed the ultraviolet light exposure and has filled the plate rack, cover the rack with a box labeled “UV light” that is provided. The box will prevent light repair by photolyase. Store at room temperature in your lab section’s bench area.
REFERENCES
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
Chess, B. (2015). Laboratory Applications in Microbiology: A Case Study Approach. New York: McGraw Hill. Cowan, M. K. (2015). Microbiology: A Systems Approach (4th edition). New York: McGraw Hill.
Electromagnetic spectrum. (2017, December 19). Retrieved from Merriam Webster Dictionary: https://www.merriam-webster.com/dictionary/electromagnetic%20spectrum
Knowledge Centre: Power of UV Disinfection. (2017). Retrieved from UV Guard: (http://www.uvguard.com/wp- content/uploads/2015/09/uv-light-spectrum-dia.jpg)
OpenStax. (2016, November 1). Microbiology. Retrieved from https://legacy.cnx.org/content/col12087/1.4
The Electromagnetic Spectrum: the family of light. (2018). Retrieved from Cyberphysics: http://www.cyberphysics.co.uk/graphics/diagrams/waves/EMSpectrumcolor.png
