7 Acid Fast Stain
The acid-fast stain is a differential stain specifically used to detect bacteria in the genera Mycobacterium and some Nocardia. They contain a waxy material in the cell wall called mycolic acid. This prevents the bacteria from being stained by different stains. (Figure 7.1) The primary stain of carbol fuchsin contains phenol, which penetrates the waxy cell wall. As with other differential stains, a positive retains the primary stain.
There are two acid-fast techniques: Ziehl-Neelsen Method and Kinyoun Method. How they differ is based on the mordant. In the Ziehl-Neelsen method procedure, the primary stain is applied over a steaming hot water bath. The steam is the mordant. By heating the mycolic acid, it further makes it more porous, letting the stain penetrate into the cell wall. The Kinyoun method does not use steam as the mordant, but an increased concentration of phenol in the carbol fuchsin. This increased amount is adequate to allow penetration of the stain into the bacterial cells, and the carbol fuchsin is not removed during the decolorizing step. This is safer since it does not release the levels of noxious phenol fumes being heat-vaporized in the Ziehl-Neelsen method, but the challenge is which technique stains more effectively than the other.
This staining technique has an important purpose. The acid-fast stain is used as a diagnostic tool in the identification of Mycobacterium tuberculosis and Mycobacterium leprae, which are the pathogens that cause tuberculosis and leprosy, respectively.
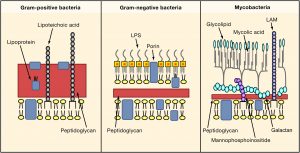
Figure 7.1 Mycolic acid is attached to the cell wall. Acid fast positive bacteria still contain peptidoglycan but less in amount compared to Gram positive and Gram negative bacteria. Image from https://www.cell.com/fulltext/S0092-8674(06)00190-5
MATERIALS
Each student should have:
Blue rack
2 glass slides
Carbol fuchsin
Acid Alcohol
Stain bottle rack: methylene blue
Lens paper
Windex (depends on instructor)
Inoculating loop
Wax pencil
Metal slide clip
Bunsen Burner
Striker
1 slant culture of Mycobacterium vaccae
1 culture of Staphylococcus epidermidis
Microscope
Share cultures between students.
PROCEDURE OF SMEAR PREPARATION
MAKE 2 SMEAR PREPS. ONE WILL BE USED AS A BACKUP.
- Draw a circle with the wax pencil on a slide.
- Flip the slide over. Write “Up”.
- Add one drop of distilled water onto the slide within the target circle.
- Using aseptic technique, transfer 1 loopful of Staphylococcus epidermidis first onto the slide, and try NOT to spread within the wax circle yet.
- Transfer small amount of Mycobacterium vaccae into the Staphylococcus epidermidis, spreading within the wax circle. Break apart clumps of Mycobacterium
- Air dry the slide. Do not apply heat to a wet slide. Do not blow on the slide.
- Heat fix the slide.
PROCEDURE OF ACID-FAST STAIN (KINYOUN METHOD)
STAIN ONLY ONE SLIDE. You may not need the second slide. This serves as a backup in case you have error in your staining procedure. It saves you time.
- Place one smear prep on a stain tray.
- Saturate the slide with carbol fuchsin for 15 minutes.
- Rinse slide with distilled water until clear over stain tray.
- Decolorize with acid alcohol for 7-10 seconds.
- Stop decolorization by rinsing with distilled water.
- Counterstain with methylene blue for 60 seconds.
- Rinse with distilled water.
- Blot dry with a paper towel.
- Examine under the microscope and show your instructor at 1000x magnification.
- After your instructor’s approval of your staining technique, dispose of your slides in the plastic beaker labeled “Self prepped” in the Discard Area.
REFERENCES
Brown, A. E. (2009). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw Hill.
Chess, B. (2015). Laboratory Applications in Microbiology: A Case Study Approach. New York: McGraw Hill.
