14 Bacterial Conjugation
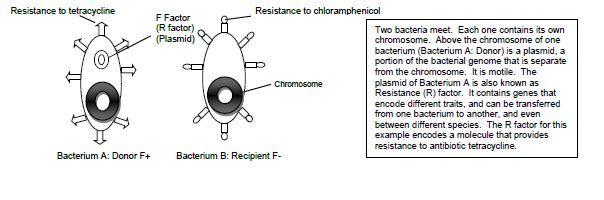
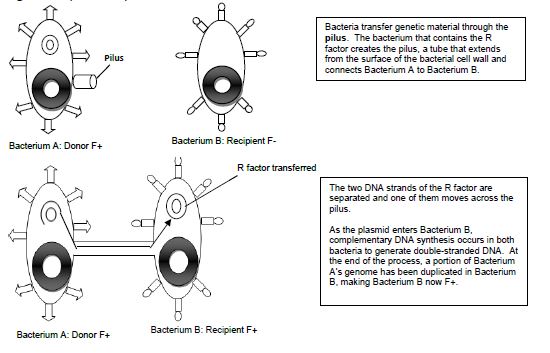
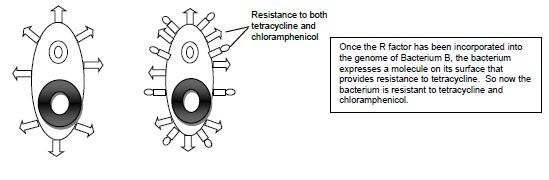
Conjugation is commonly known as bacterial “sex.” It is a mode of genetic exchange in which a plasmid or other genetic material is transferred by a donor bacterial cell to a recipient bacterial cell via a direct connection. Both Gram positive and Gram-negative bacteria cells can conjugate. The donor has a plasmid, known as F factor (F = fertility), that allows the synthesis of a conjugative pilus. The recipient cell has a recognition site on its surface for the pilus. The pilus is a tube that extends from the surface of the bacterial cell wall and connects two bacteria, therefore making a direct connection. The bacteria involved in conjugation are denoted by either F+ or F-. F+ is for the donor cell that has the F plasmid, and F- is for the recipient cell that lacks it. After the two bacteria are attached by the pilus, one strand of DNA of the F plasmid moves across the pilus from the F+ cell to the F- cell, and DNA synthesis occurs in both bacterial cells to generate double-stranded DNA. At the end of the process, the recipient bacterial cell has a portion of the donor bacterial cell’s genome and can express traits that were on that plasmid in the donor cell.
Examples of conjugation are antibiotic resistance of bacteria, chemical tolerance, and ability to use new metabolites. Special resistance (R) plasmids, or factors, that bear genes for resisting antibiotics and other drugs are shared among bacteria through conjugation. This course will be carrying out an experiment of the example of antibiotic resistance by demonstrating the transfer of the R factor which has resistance to the antibiotic tetracycline between two subspecies of E. coli, Top10F and JW0157. The donor F+ cell (Top10F) is resistant to tetracycline, which is found on a F factor plasmid, and the recipient F- cell (JW0157) is resistant to chloramphenicol. (Figure 14.1). Ahead of class time, both subspecies were grown separately overnight in media enriched with the appropriate antibiotic at 37°C. The following day each culture was diluted by 1:100 dilution into media without any antibiotics and placed into a dry shaker at 37°C, where they were vigorously shaken until they reached the middle of exponential growth phase.
Figure 14.1 Schematic drawing of bacterial conjugation.
MATERIALS
Optional for instructor:
4-5 ml serological pipettes
Green pi-pump
Per group of three students should have:
Wax pencil
3 LB + tetracycline (10 μg/ml) & chloramphenicol (25 μg/ml) plates
(LB = Luria-Bertani)
3 Sterile cotton swabs
PROCEDURE OF BACTERIAL CONJUGATION
The following procedure should be performed by the instructor:
- Fill three Petri plates with each of the cultures as follows:
a. Donor only
b. Recipient only
c. Donor + recipient (equal volumes of each)*
*Note: The volume of each culture depends on the size of the petri plate. The volume should be enough so that entire bottom of Petri plate is covered and liquid culture will agitate when the plate is placed onto a platform shaker. - Agitate cultures on a platform shaker (not too fast) at room temperature between 30-90 minutes.
To be performed by the students in groups of 3:
- Label the bottom of the LB + tetracycline & chloramphenicol plates with student names and date. Label one plate with one cultural scenario: Donor Only, Recipient Only, and Donor + Recipient.
- Each student is responsible for one scenario: Using a sterile cotton swab, make a lawn of bacteria from each broth culture onto a LB + tetracycline & chloramphenicol plate.
Incubate at room temperature in your lab section’s racks.
REFERENCES
Cowan, M. K. (2015). Microbiology: A Systems Approach (4th edition). New York: McGraw Hill.
Waldburger, Carey. (2008). Conjugation protocol. Wayne, NJ: William Paterson University.

